Questmed GmbH - German accredited Test Laboratory
Questmed GmbH offers biomechanical laboratory services to medical devices manufacturer concerned with the fields of joint replacement, osteosynthesis, dental implants and cardiac devices. We are specialized in static and dynamic mechanical testing of implants and we possess experience for decades. We are adept in almost every tests regarding fatigue and wear of implants, their analyses and documentation.The Questmed GmbH was founded in 2008 in Greater Berlin. Since 2018 we are also established in Jena.
Accreditation and Recognition - Certificates
Questmed GmbH is an german accredited test laboratory for medical devices according Directives 93/42/EEC and DIN EN ISO/IEC 17025:2018.Questmed GmbH is an recognized testing laboratory according to § 15 (5) of the German Medical Devices Act for medical devices.
In the medical devices sector the international agreements have currently been made with the European Community with regard to the mutual recognition of conformity assessment with United States of America, Australia, New Zealand, and Switzerland as well as ILAC MRA.
Standardization Activities
Questmed is active expert and member of national and international standardization working groups (DIN, ISO, ASTM) for medical devices.These standards helps to increase the patient safety by comparability and reproducibility of medical device performance.
We are active working on standards for cardiac Occluders (ISO/DIS 22679), cardiac Stents (ISO 25539, ISO/DTS 17137, ASTM F2477, ASTM WK61103), dental Implants (ISO 14801, ISO/CD 22683, ISO/WD 3843, ISO/WD 24230), Ion Release Evaluation of Medical Implants (ASTM F3306).
Christian Abicht
Christian Abicht (Dr.rer.med. Dipl.-Ing.) is the CEO of Questmed GmbH. He was general manager and quality manager of the Questmed GmbH for many years. He studied electrical engineering, received his PhD in Anatomy and worked as procuct development manager for medical devices.Test Equipment
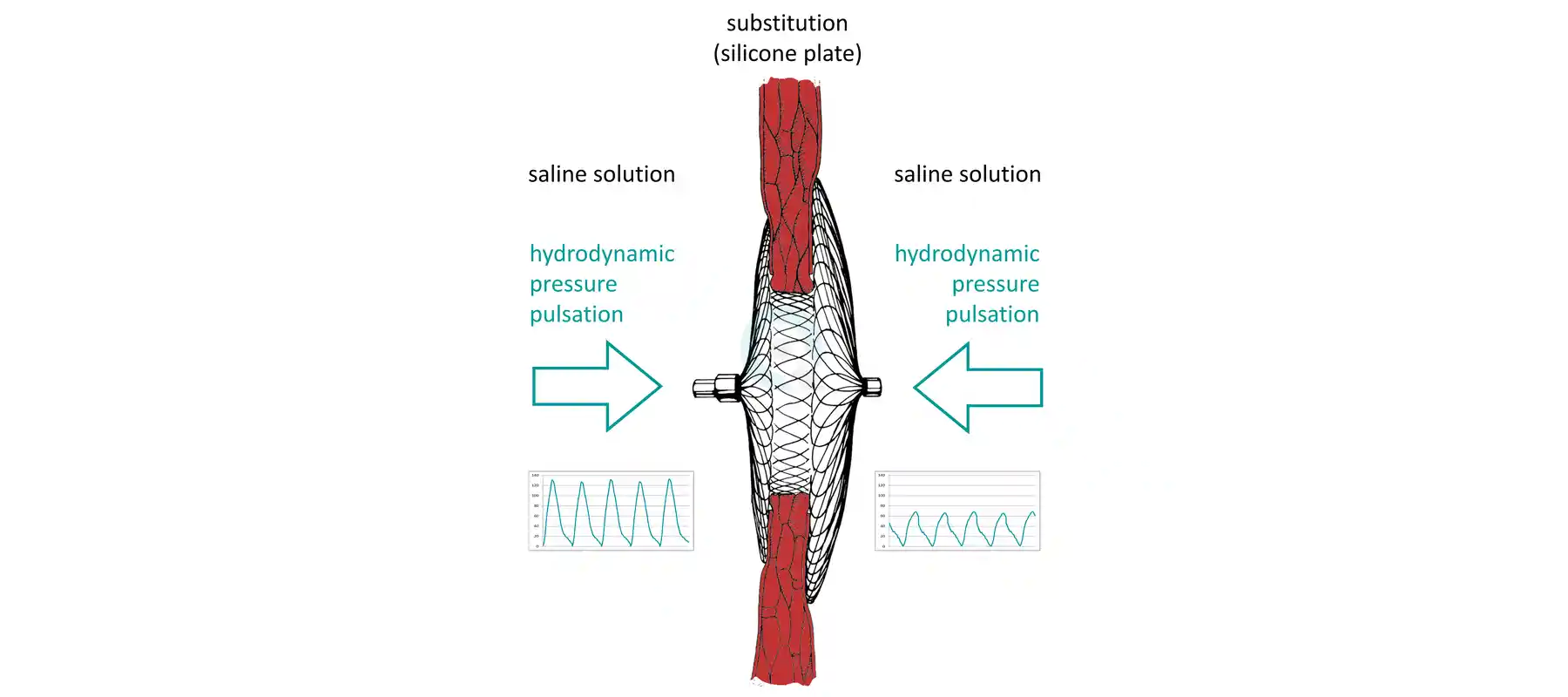
The test equipment of Questmed GmbH based on advanced technology. Moreover, they were adaptet to the latest standards constantly and continuously enhanced. The tests aim at the verification of the physical resistance of implants in conditions almost as in-vivo.We are convinced that physical testings of medical devices improve the security of implants for patients and furthermore that they may contribute to coast reduction in health care service.
We are running one highdynamic multiaxial test system, 17 dynamic test systems up to 10 kN, one knee wear simulator with two banks of three test stations and six multidevice cardiac implant test systems.